Stability Chamber Validation Services
TURN-KEY, COST-EFFECTIVE TEMPERATURE AND HUMIDITY MAPPING VALIDATION SOLUTIONS
Parameter has extensive experience in the development and execution of industry-proven stability chamber validation protocols. Parameter is equipped to provide and execute stability chamber validation protocols such as Installation, Operational and Performance Qualification.
We collect all temperature and humidity data using high-quality equipment to ensure precise measurements at each mapped point within the chamber. Our validation process ensures that all equipment used is calibrated and traceable to national standards, providing reliable and accurate results for stability chamber validation.
TEMPERATURE MAPPING VALIDATION DOCUMENTATION
Parameter provides a complete document package tailored to your needs with each stability chamber validation and temperature mapping validation. We can produce a comprehensive stability chamber validation protocol for you or follow a protocol that you have already created. If you don’t need a full protocol for your temperature and humidity mapping study, we can provide a basic stability chamber validation plan prior to performing the study to specify the mapping details.
Regardless of the level of documentation you choose, after the stability chamber validation and mapping study has been completed, you will be provided with a clear, comprehensive summary of the results.
Document Package results include:
- Minimum, Maximum and Average Statistics
- Mean Kinetic Temperature calculations
- Detailed graphs
- Test data
- Deviations
- The executed protocol
- Test equipment calibration data with NIST-traceable calibration certificates.
Chamber Mapping studies include:
- IQ/OQ/PQ
- Empty Chamber Mapping
- Full / Loaded Chamber Mapping
- Simulated Power Outage
- Open Door Recovery
- Alarm Testing
- Single and Multi-point Conditions
- Calibration Verification
Once execution is complete, a stability chamber validation report package is carefully prepared, reviewed and submitted for client approval.
Our Validation Engineers have extensive experience with temperature and humidity stability chambers and can provide you with the high quality service that will assure sample integrity and satisfy the most stringent regulatory requirements. Our ultimate goal is to leave you with the peace of mind that your company’s validation requirements are being met.
FAQs About Stability Chamber Validation Services
What is stability in validation?
Stability in validation ensures that a controlled environment (like a stability chamber) consistently maintains specified conditions over time to support reliable product stability studies. This process typically includes:
- Temperature and Humidity Control: Verifying consistent maintenance of specified temperature and humidity levels.
- Calibration and Mapping: Regular sensor calibration and temperature/humidity mapping for uniformity and accuracy.
- Compliance with Standards: Adherence to industry guidelines (e.g., FDA, ICH) to meet necessary standards.
- Documentation: Thorough documentation of all validation activities and results to demonstrate compliance and reliability.
What are the requirements for a stability chamber?
A stability chamber typically requires the following to ensure reliable and compliant operation:
- Precise Temperature Control:
Ability to maintain temperature ranges often from -40°C to +60°C.
Accuracy within ±0.1°C and uniformity within ±0.5°C.
- Accurate Humidity Control:
Capability to maintain humidity levels from 10% to 95% RH.
Accuracy within ±1% RH and uniformity within ±2% RH.
- Regulatory Compliance:
Adherence to ICH Q1A (R2), FDA, and GMP guidelines.
Regular validation and calibration.
- Uniformity and Stability:
Consistent environmental conditions throughout, verified by mapping.
- Robust Construction:
Durable materials (e.g., stainless steel) for long-term reliability.
- Data Logging and Monitoring:
Systems for continuous recording, real-time monitoring, and remote access.
Alert notifications for deviations.
- Safety Features:
Alarms for temperature, humidity, power failures, etc.
Backup systems for uninterrupted operation.
- Thorough Documentation:
Comprehensive records of validation, calibration, and monitoring.
Reports and certificates for compliance and audits.
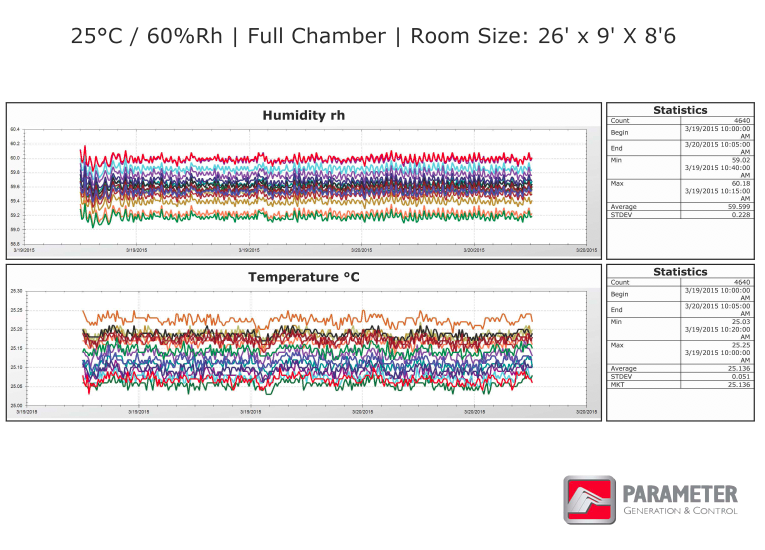
STABILITY CHAMBER QUALIFICATION GRAPHS
These graphs show stability chamber validation data performed on a large walk-in room, once while empty and again while full. Each color represents a different sensor located throughout the room. Notice the standard deviation rates for the entire data set represented on the right of the graphs, indicating that the walk-in room is being controlled very tightly throughout the stability chamber validation cycles.